The tl;dr:
We have an application form (well, two if you want to be technical about it). It's designed to meet the requirements of the National Statement. But we also made it so that it's easy to fill out if you've already got the information somewhere else (like your research/evaluation plan).
Introduction
One of the biggest challenges we hear from the sector when it comes to ethical approval in any form, is the forms. Not only are there many forms to complete, many of them require duplication of information from existing documents. And to add to the challenge, many forms are designed for medical and clinical research and use terminology that can be confusing or alien to evaluators and market and social researchers.
It makes sense. The ethics system in Australia and overseas originated from a need to ensure safety for participants in medical and clinical research settings, and to enable resource-constrained institutions to make efficient decisions. As people have recognised that other sectors need similar protection, the ethics system has expanded. In principle this expansion is a good thing, but in practice, there are points of friction that arise in the system.
Less can be more
A key goal for Iris Ethics in setting up our HREC was to do what we could to reduce the friction in the system for applicants. Part of that was reducing the paperwork needed to submit an application. Our rationale and experience is that if you've written a high quality evaluation or research plan, then it should have almost all the information needed to enable us to make an assessment. If that information isn't there, then it's our duty to follow this up with you as part of the application process.
Nonetheless, we still need to get you (and your principal investigator or project director) to fill out a form or two. But we've tried to make them as short and as clear as we can, while still ensuring that we meet the requirements of the National Statement. We've also made the decision to say (where possible) that you can refer us to existing documents that provide the information, rather than have you copy things across. And we've created this guide to help you navigate each of the forms and to help you get your information ready before you submit.
Form 1: The main application form
You'll encounter this form when you've set up an account with us, selected the type of review product, and then go to "check out".
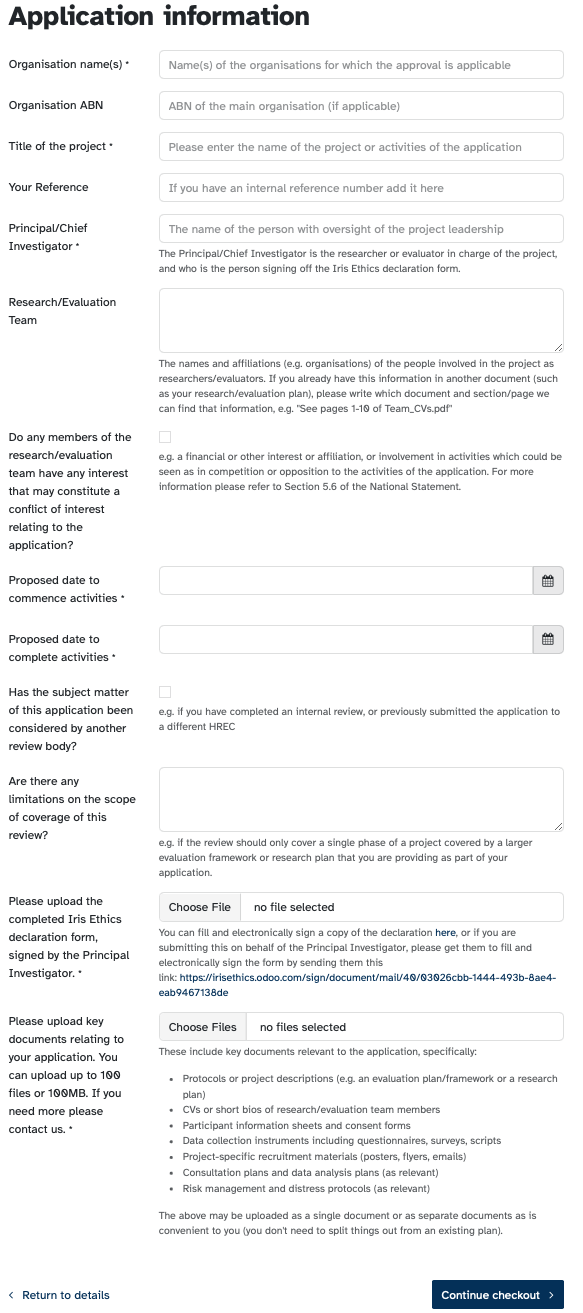
Some of the fields are pretty straightforward (like the name of your organisation and your project) and we've added explanatory text where we can. But we've got a little more context for a couple of the questions:
- Conflict of Interest: Conflict of interest can be a complex topic, and we've added it to the list of future resources to develop. If you click yes to this, you'll be able to add more detail on the nature of the interest (or refer us to a document that discloses this). Just because it's declared doesn't mean that it's a conflict of interest, but it does give us information that we can consider as part of the decision making process, and allows us to help ensure that risks are managed. The National Statement at Chapter 5.6 talks broadly about the meaning of conflict of interest, but we've added some non-exhaustive examples of declarable interests from our sectors:
- An evaluation team is evaluating a grant program, but they are also evaluating one of the grantee projects
- A market researcher has shares in the commercial client
- A social research team has long-standing personal relationships with research stakeholders that may influence the recruitment process
- Consideration by another review body: This could mean a full ethics review with another HREC, or an internal peer review before sending to us. We just want to make sure that we aren't duplicating review. If you select this, you'll be able to provide more information.
- Limitations on scope: Many projects have multiple streams of activity that are separable in the context of ethical review. A process evaluation of a program may only need a lower risk review, while the outcomes evaluation of the same program needs a full review. Or a research plan may commence with a literature review (which may not need ethics review at all) before moving to direct data collection (which does). Telling us the scope of the review gives us a sense of where to focus our attention.
Then there's the declaration form.
Form 2: The declaration
This is the longer form. But from an ethical standpoint it's the most important. We need to make sure that there is a commitment to upholding the principles of the National Statement, expressed by the Principal Investigator (a.k.a. the person in charge of the project). So the form has to capture not only a formal declaration that the project is aware of and seeks to meet ethical standards, it has show evidence of how that is achieved. It's not a substitute for our review and decision-making, but it's a good way to think through your project and ensure that you have covered off key areas as part of your application.
You can even take a look at the form now.
The good news:
- It's electronically signable. No printing out needed.
- The link is shareable, so if you're not the Principal Investigator you can send it to the person that is in that role to complete and sign. Once they've signed it they can email it to you and also download a copy for your records and to upload when submitting the application.
- We've kept the number of questions short, and you can refer to documents such as your research plan to make it easier.
- Each question reflects the principles of the National Statement:
- Merit and integrity
- Justice
- Beneficence
- Respect
The bad news:
There isn't any (well, we don't think there is). If there is any part of it that's confusing, contact us. It's probably a sign that we need to improve it, or make a resource to help everyone.
Uploading files
The last part of the application is to upload files related to your application. We've provided a list of the kinds of information these documents should have. In some cases, the information might be in the same file (no point uploading your interview instruments separately if they're already in the research plan). If you think the document shows how you are managing ethical risks in your project, then it should probably be uploaded. But you don't need to copy and paste from your documents if the information is already there. You can just refer to them in the declaration and other parts of the form.
Summary
That's our forms. If we make changes, we'll also update this guide, so that you have the most up to date information. But our goal is to make it as easy as possible to get ethical review for your projects. The less time spent filling out forms is more time you can spend ensuring that your work is ethical, and that's what we really care about.